Research Summary
The ultimate goal of research in my independent laboratory is to develop cutting-edge single-molecule biophysical technologies and establish non-equilibrium physics models to understand the molecular mechanisms underlying functional biomolecular dynamic clusters in molecular biology. Our research focuses on two types of biomolecular dynamic clusters: dsDNA-protein co-condensation and ssDNA-RPA complex. These studies have the potential to lead to the development of new strategies for intervening in human diseases.
1) Deciphering the molecular mechanism of dsDNA-protein co-condensation
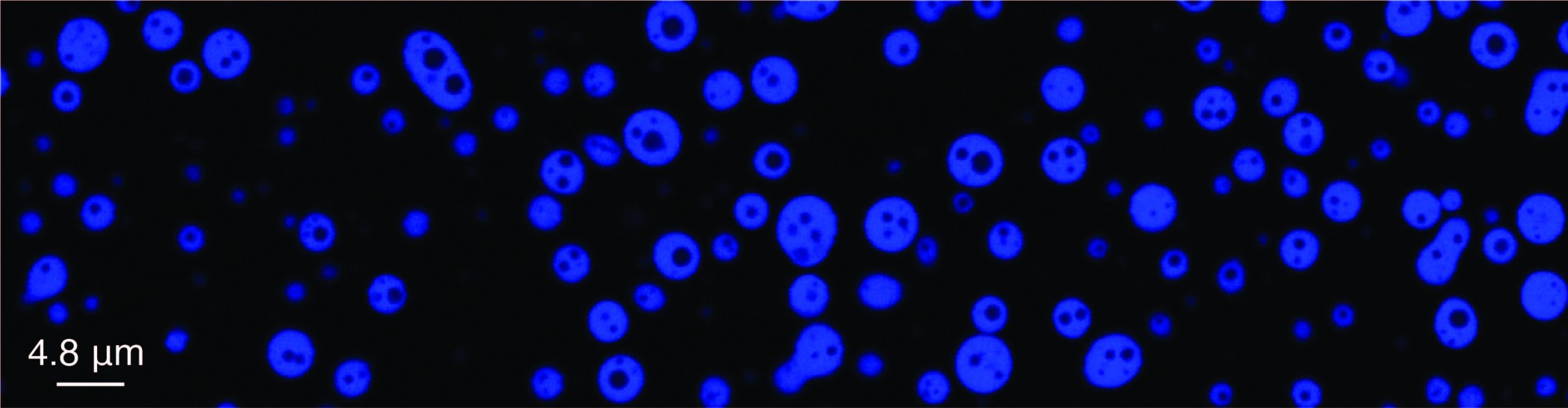
Eukaryotic cells utilize lipid membranes to compartmentalize their intracellular organization. However, recent in vivo and in vitro studies have revealed that these cells also employ an alternative strategy for organizing their complex biochemistry. For instance, macromolecules such as nucleic acids and proteins can undergo liquid-liquid phase separation (LLPS) to assemble, resulting in the formation of membrane-free, biomolecular dynamic clusters. The major driving forces for LLPS are protein intrinsically disordered regions (IDRs) and/or multivalent interactions between modular biomacromolecules. These functional clusters play crucial roles in several essential biological functions, while aberrant clusters can lead to various human diseases.
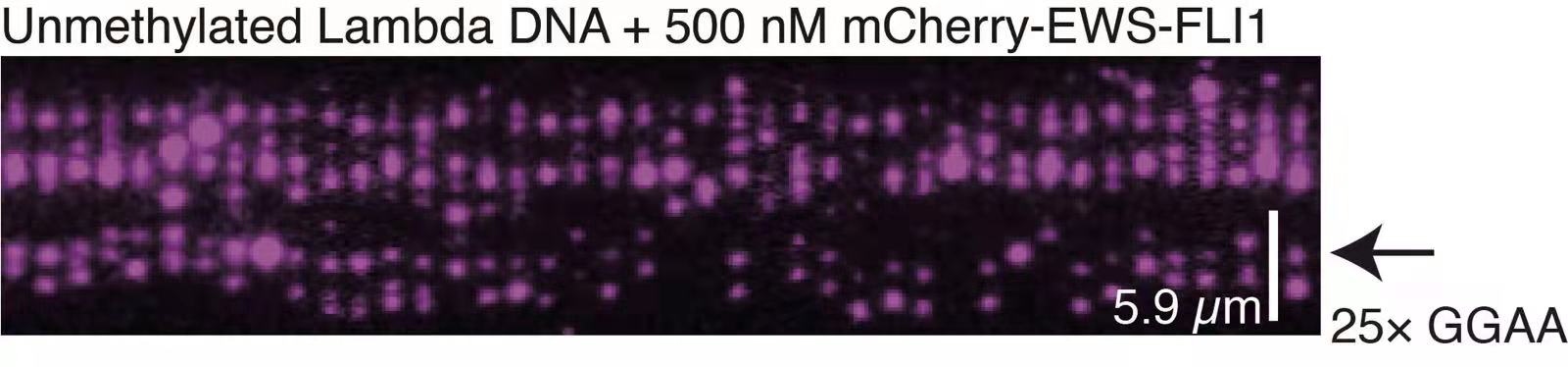
Functional biomolecular dynamic clusters can be formed by individual forms of biomacromolecules or multiple types, identified as single-component condensates or multi-component condensates, respectively. For instance, dsDNA has been found to act as scaffolds for the formation of dsDNA-protein co-condensation. Understanding the molecular mechanism underlying these co-condensations is crucial for deciphering vital cellular processes, such as chromatin formation and transcription initiation condensate formation. Although several studies have focused on dsDNA-protein co-condensation, challenges have arisen in comprehending the underlying principles of co-condensation formation and function. To address this issue, we utilized newly developed single-molecule techniques and non-equilibrium physics to decipher the molecular mechanism of dsDNA-protein co-condensation.
2) Investigating the molecular mechanism of ssDNA-RPA complex
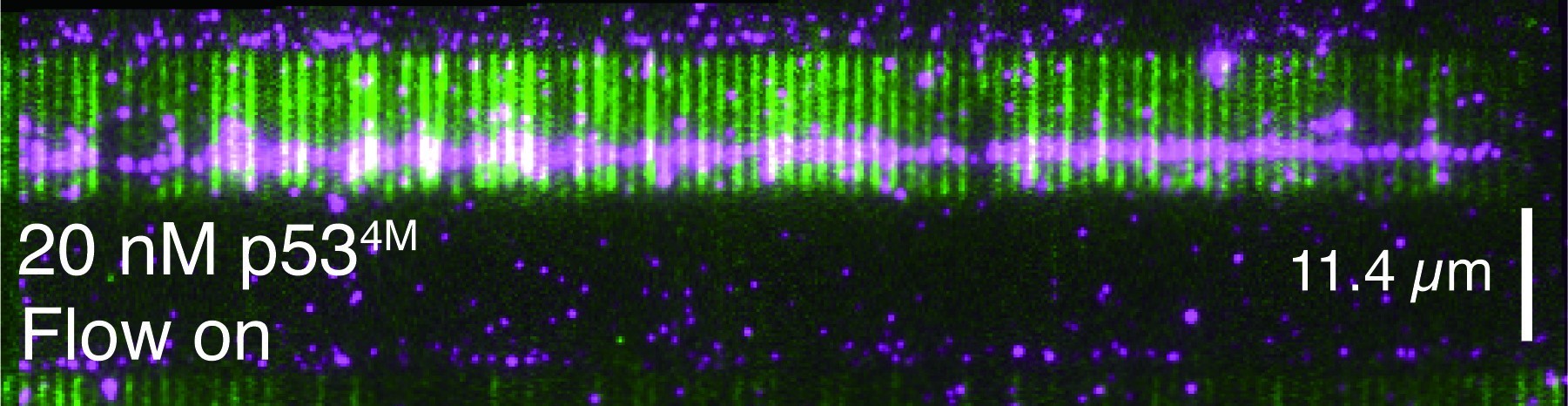
Replication Protein A (RPA) is the major single-stranded DNA (ssDNA)-binding protein in eukaryotes. It acts as the first responder to ssDNA exposure and coordinates downstream DNA metabolic pathways, such as DNA replication and repair. In humans, mutations in RPA have been associated with various diseases, including breast and colon cancer. During DNA replication, RPA participates in initiation, lagging strand synthesis, and replication checkpoint activation. In DNA repair, RPA is crucial for nearly all repair pathways involving ssDNA intermediates, including mismatch repair, nucleotide excision repair (NER), and homologous recombination (HR)-mediated DNA double-strand break (DSB) repair. Although the function of RPA can be modulated by RPA-interacting proteins (RIPs) and post-translational modifications in response to DNA damage, the precise mechanism by which RPA directs the choice of DNA metabolic pathway for the same ssDNA bound remains to be elucidated.
RPA in Saccharomyces cerevisiae is a heterotrimer composed of three subunits: Rfa1, Rfa2, and Rfa3. Each subunit contains at least one oligonucleotide/oligosaccharide-binding fold (OB), also known as the DNA-binding domain (DBD). Rfa1 has four OB folds (DBD-F, DBD-A, DBD-B, and DBD-C), Rfa2 has one OB fold (DBD-D), and Rfa3 has one OB fold (DBD-E). Additionally, Rfa2 has a winged helix-turn-helix (WH) domain at its C-terminus. Currently, it is believed that the WH domain and DBD-F are not involved in ssDNA binding but rather in protein-protein interactions. Despite each DBD having weak affinity with ssDNA (with a dissociation constant in the micromolar range), a cooperative action among the DBDs enables RPA to bind ssDNA tightly, with dissociation constants as low as sub-nanomolar levels. Multiple RPA molecules bind to long ssDNA, forming an ssDNA-RPA complex. This complex has recently been shown to be another type of membrane-free, functional biomolecular dynamic cluster. We utilized newly developed single-molecule techniques and non-equilibrium physics to investigate the molecular mechanism of ssDNA-RPA complex.